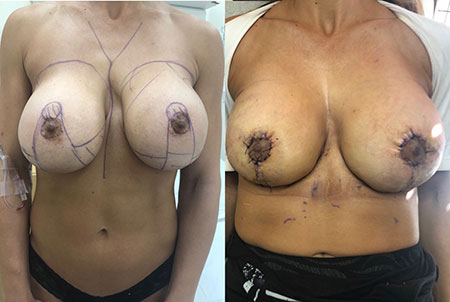
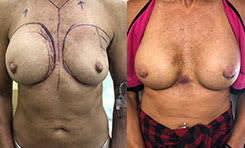
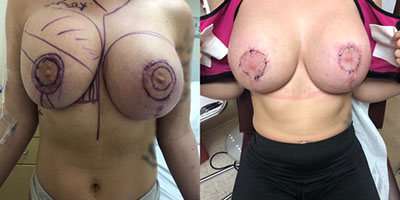
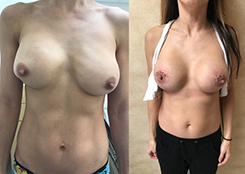
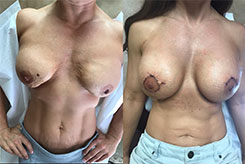
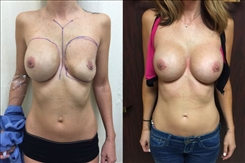
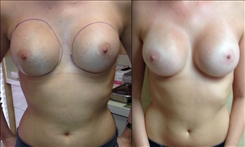
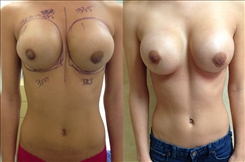
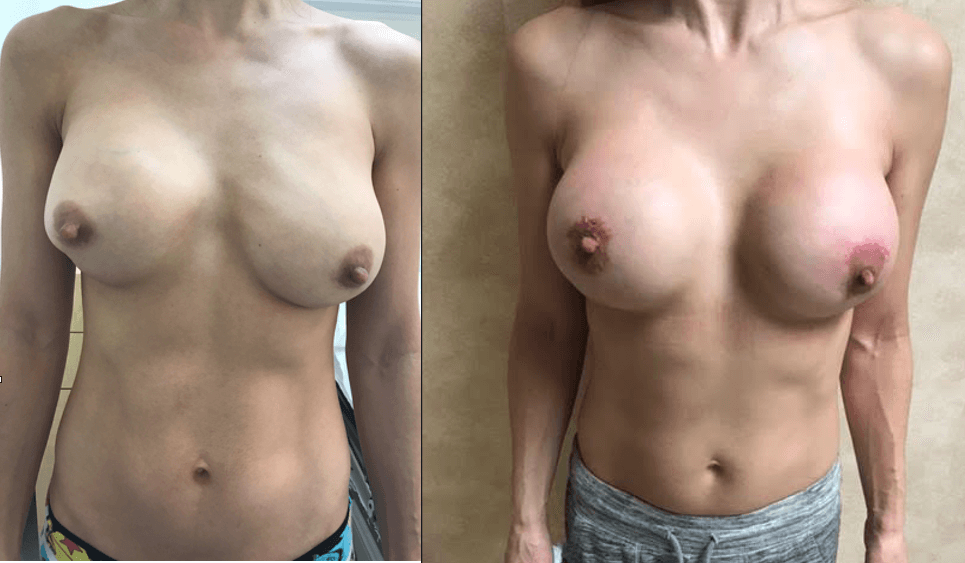
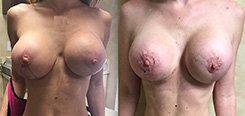
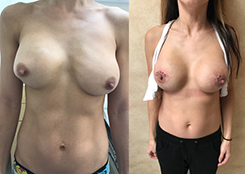
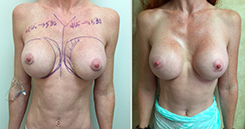
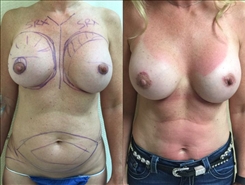
Breast Revision
Breast implants are designed for long-term breast enhancement; however, changes in aesthetic desire or certain circumstances dictate they must be replaced with a procedure commonly known as breast revision surgery or secondary breast augmentation.
Whether breast augmentation has resulted in damaged implants or an unsatisfactory breast appearance, Beverly Hills Plastic Surgeon Dr. Stuart A. Linder can revitalize the look of your breasts with breast revision. Dr. Linder has been performing breast enhancement surgeries since 1997 and has completed over 1,000 breast revisions.
During your private consultation, Dr. Linder will evaluate your needs and desires to help determine the best technique to achieve your specific aesthetic goals. Women choose breast implant revision surgery to:
- Resize their implant
- Correct implant complications
- Change the placement of their implants
- Remove implants permanently

Ideal Candidates
Ideal candidates for Beverly Hills breast implant revision are interested in removing or replacing their breast implants for health or aesthetic reasons. These women have previously undergone breast surgery and have completely healed from that surgery. Candidates should be in good overall health, not smoke, have realistic expectations, and have a positive outlook regarding the surgery.
Contrary to popular belief, breast implants do not need to be replaced every 10 years. If you are free from pain and happy with how your breasts look, there is likely no reason for breast revision; however, it is recommended to see a board-certified plastic surgeon if you have any concerns regarding the condition of your breast implants.
Candidates who no longer want breast implants can choose implant removal without replacement.
Reasons to Consider Breast Revision
Dr. Linder prides himself on educating his patients throughout the process, beginning with the consultation and extending all the way to the last follow-up appointment.
Are you Ready for A Breast Revision?
Natural Changes
Breasts tend to change over time in terms of laxity, size, and shape, regardless of whether they were previously operated on. Pregnancy, extreme weight fluctuations, and aging can all affect how the breasts look, even after augmentation. Breast revision addresses the natural changes in the breasts after augmentation and can give you a more pleasing chest appearance.
Post-op Complications
Many of the potential complications associated with breast augmentation are uncontrollable. For example, implant failure or capsular contracture may occur even in patients who received exceptional care during their breast augmentation treatment. Breast revision can correct postoperative complications, such as implant rupture, and restore a pleasing bustline.
Unsatisfactory Results
Personal preferences regarding breast aesthetics can change through the years, and sometimes the outcome of an initial breast augmentation is unsatisfying right away. When breast augmentation does not meet a patient’s expectations, it is appropriate to consider breast revision. Implant size, placement, and type can be modified during breast revision.
Common Breast Concerns Addressed With Revision
Capsular Contracture
Scar tissue formation is a natural response to foreign objects in this body. This scar tissue capsule forms around the implant and often causes no problem. However, in some circumstances, the tissue hardens, causing pain and an irregular breast appearance. Capsular contracture is corrected through one of the following approaches:
Capsulotomy: This procedure involves expanding the capsule, softening the breast, and giving it ample room.
Capsulectomy: This procedure involves removing the capsule altogether, which may reduce the chance of recurrence.
Common Symptoms
- Painful scar tissue
- Visible distortion
- Cold and hardness to the breast
Double Bubble Breast Deformity
Double bubble breast deformity occurs when the implant is improperly placed during breast augmentation. It is quite common with the transaxillary and transumbilical approaches due to the long distance between the incision and surgical site. To correct double bubble deformity, Dr. Linder repositions the implant and performs a breast lift to reshape the breast.
Common Symptoms
- Elevated implant
- Skin looseness of the lower breast
- Often painful scar tissue
Anatomical Breast Implant Deformity
Because anatomical breast implants are not round, any rotation in the breast pocket causes deformities that can only be fixed with breast revision. During revision, anatomical implants are often replaced with round implants, and capsulectomy may also be required to produce optimal results.
Common Symptoms
- Rotational deformity
- Postoperative implant movement
- Misshapen breast
Bottoming Out
Bottoming out occurs when implants are malpositioned during breast augmentation or when the weight of the implants weakens the lower portion of the breast. Several techniques may be implemented to repair breast implants that have bottomed out. It is a challenging irregularity to mend, but Dr. Linder is happy to apply his breast revision expertise to help his patients achieve the breasts they desire.
Common Symptoms
- Elevated implant
- Skin looseness of the lower breast
- Often painful scar tissue
Implant Rippling
Implant rippling and visibility can occur both above and below the muscle. Placing silicone or saline implants above the muscle in the subglandular retromammary plane does increase the risk of visibility around the medial breast, as there is no muscle coverage. However, even patients who are extremely thin may develop visibility and rippling around the medial breast.
Ruptured Implants
Ruptured implants are a relatively common reason for Beverly Hills breast revision surgery. When implants rupture, it is important to have them replaced as soon as possible to avoid excessive scarring in the breasts. If too much scar tissue has accumulated around the deflated implant, it becomes difficult to create a normal breast shape in the future.
Common Symptoms
- Painful to touch
- Visible asymmetry
- Loss of integrity to the shell
Desire for Different Implant Size
Personal preferences regarding breast implant size can change over the years. Many patients who undergo breast revision decide to exchange their implants for a different size or remove them altogether. Removal is often paired with a breast lift in cases where previous implants have caused skin laxity.
How Much Does Breast Implant Revision Cost in Beverly Hills?
Many factors are considered when determining the cost of a breast revision surgery, the most significant variable being the reason for revision. Post-op complications (capsular contracture, double bubble, bottoming out, ruptured implant), a desired implant size change, and the type of implant being replaced must be evaluated prior to determining the cost. Additional factors include surgeon fees, facility fees, and anesthesia fees. You will receive a cost estimate for your procedure during your initial consultation.
To get a more accurate cost estimate, please contact our office for a consultation.
Contact UsBreast Implant Revision Surgery Information:
Where Is My Surgery Performed?
Dr. Linder performs most breast implant revision surgeries at Summit Surgical Center in Beverly Hills, California. The surgery center is fully accredited and certified by the American Association for Accreditation of Ambulatory Surgery Facilities, Inc., and Medicare.
He also has privileges at the premier medical center Cedars-Sinai in Los Angeles and has been an Attending Surgeon in the Plastic and Reconstructive Surgery Division since 1997.
What Type of Anesthesia Is Used?
Breast revision surgery at Summit Surgical Center is performed under general anesthesia and administered by a board-certified anesthesiologist. While a laryngeal mask is typically used, patients with a history of gastroesophageal conditions may opt for endotracheal tube intubation.
What Brands of Implants Are Used at This Facility?
Dr. Linder prefers smooth, high-profile saline and silicone implants by either Mentor® or Allergan™. Both are FDA approved.
What Are the Potential Risks Associated With Breast Revision Surgery?
Complications related to breast revision surgery are uncommon, but there are risks to any surgery. Some of these risks include:
- Changes in sensation
- Hematoma
- Scarring
- Unsatisfactory results
How Long Does Breast Implant Revision Take?
Each breast revision is different depending on the cause and the techniques used. Therefore, the length of the surgery will vary. Usually, this surgery will take between one and three hours to complete. This does not include pre-op or observation after the surgery.
What Surgeries Are Performed in Combination?
The most common combination with breast implant revision is breast lift surgery. Many women choose this combination to restore a youthful, perky appearance. This combination is especially beneficial if you decide not to replace your implants or if you are replacing the implants for a smaller size.

Your Consultation
Dr. Linder understands that you have many questions and concerns regarding your breast revision surgery.
These questions and concerns can be addressed during your consultation. Whether you are a new patient or Dr. Linder performed your initial breast augmentation, this meeting will give you a chance to discuss your concerns and what you hope to achieve with your revision.
During your consultation, Dr. Linder will:
- Examine your current implants for any noticeable complications
- Take measurements
- Review your medical history
- Verify the correct techniques for your needs
- Discuss combination procedures, such as a breast lift
Breast Implant Revision Surgery Timeline
Preoperative
An intravenous antibiotic is given before your breast revision.
Surgery
Breast revision is performed under general anesthesia. The length of the procedure will depend on the techniques required and whether a breast lift is being performed simultaneously.
Recovery Room
Federal law requires you to remain in the recovery room for at least one hour of observation following your breast surgery.
After Surgery
You will be sent home with dressings and instructed to take oral antibiotics and pain and nausea medication for the first week. Breast revision patients should avoid heavy lifting or raising the arms above the head for at least three weeks or when cleared by Dr. Linder.
Postoperative Visit
Surgical dressings are removed. You will be recommended to wear a compression bra, such as the Dr. LinderBra™. The LinderBra™ utilizes a double-clip zip mechanism, allowing easy placement with fresh gauze.
Day 7 Follow-Up
Dr. Linder examines your breasts for infection.
Days 8 to 14
Replace your dressings twice a day, and be sure to keep your incisions clean and dry.
Day 14
All sutures will be removed
Day 21
Dr. Linder will determine if light activities may be resumed.
Day 28
Daily activities can usually be resumed, and regular bras can be worn.

After Your Revision
What Is the Normal Recovery Time for a Breast Implant Revision?
Most women experience a similar recovery to their initial breast augmentation. During this time, be sure to follow all of Dr. Linder’s post-op instructions and avoid any heavy lifting or strenuous activities.
What Types of Medications Will I Be Given After My Surgery?
Dr. Linder usually prescribes one of two antibiotics: Ciprofloxacin or Keflex, which is not ideal for patients allergic to penicillin. Additional pain management medications, such as Norco or Vicodin, may also be given as well as Zofran to prevent nausea.
Will There Be Scarring After Breast Revision Surgery?
In most cases, Dr. Linder can use the same incisions as your original breast augmentation. Because of this, patients tend to have minimal additional scarring after breast revision. Occasionally, additional incisions must be made, or original incisions must be lengthened, depending on the techniques used. Fortunately, these scars can still be hidden with bras and bathing suits.
All scars will fade over time.
What Can I Do to Help Minimize Scarring After Surgery?
Women can help minimize the visibility of their scars by following these steps:
- Avoid sun exposure on the incisions for up to one year
- Keep the incisions clean for at least two to three weeks post-op, as this will help prevent infection
- Keep incisions dry for at least 14 days — avoid getting the incisions wet or sweating, as this can lead to bacterial infections
When Can I Return to Normal Activities After Breast Implant Revision?
The length of your recovery depends greatly on the extent of correction required and the techniques used during breast revision. Usually, patients can go back to work within one week, but any strenuous activity should be avoided for at least three to four weeks.
How Long Will the Results of Breast Revision Last?
Breast revision results are considered permanent; however, the natural aging process will likely show with the breasts eventually. Additional implant complications are also possible, and patients should continue monitoring their implants.
Why Choose Dr. Linder for Breast Revision Surgery
Stuart A. Linder, M.D., is a board-certified plastic surgeon in Beverly Hills, California. He has over 25 years of experience performing breast revision surgery.
Dr. Linder prides himself on educating his patients throughout the process, beginning with the consultation and extending to the last follow-up appointment. Patients from all over the world come to Beverly Hills to see Dr. Linder and have him perform their breast revision.
Dr. Linder understands that patients will have many concerns and questions about their breast implant revision. Please contact our office in Beverly Hills; our friendly staff will be happy to provide you with more information.